Separation Techniques
Basis for separation- different component different properties
Strategy: devise a process that discriminates between components with different properties
Separation
Components in a mixture retain their identities
The more similar the properties are, the more difficult it is to separate them
Basis Techniques
Filtration
Floatation
Crystallization and Extraction
Distillation
Chromatography
Hand separation and Evaporation- Boil away the liquid and the solid remains (solid to solid)
A mechanical mixture of heterogeneous mixture can be separate by using a magnet
Filtration (solids (not dissolved) and liquids)
-Pass a mixture through a porous filter
Crystallization (solid in liquid)
-Precipitation, solids are then separated by filtration or floatation
Saturated solution of a desired solid
Evaporate or cool- solid comes out as pure crystal
Gravity (solids based on density)
-A centrifuge whirls the test tube around at high speeds forcing the denser materials to the bottom. Work best for small volume.
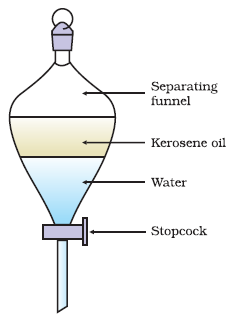
Solvent Extraction
-Mechanical mixture- use liquid to dissolve one solid but not the other so the desired solid is left behind or dissolved
Solution- solvent is insoluble with solvent already present
Distillation (liquid in liquid solution)
-Heating a mixture can cause low boiling components to volatilize (vaporize)
Chromatography
-Flow the mixture over a material that retains some components more than others, so different components flow over the materiel at different speeds
-Sheet
Paper Chromatography (PC)
-stationary phase is liquid soaked into a sheet or strip of paper
-components appear as separate spots spread out on the paper after drying or “developing”
Thin layer chromatography (TLC)
Stationary phase is a thin layer of absorbent (Al2O3 or SIO2, usually coating a sheet of plastic or glass)




In stock products can be shipped out within 3-5 business days upon receipt of customers' purchase order. 1-decyl-3-methylimidazolium tosylate
ReplyDelete