Around 460 B.C.E. a Greek philosopher Democritus first questioned the existence of atoms. He thought the atom was a sphere with barbs on the surface that held it together with other atoms. Not until about the 1800's though did the idea of the atom become revised. John Dalton was the first modern scientist to look at the fact that matter was made up of atoms.
Tuesday, 3 April 2012
History of the Atom
History of the Atom
Around 460 B.C.E. a Greek philosopher Democritus first questioned the existence of atoms. He thought the atom was a sphere with barbs on the surface that held it together with other atoms. Not until about the 1800's though did the idea of the atom become revised. John Dalton was the first modern scientist to look at the fact that matter was made up of atoms.
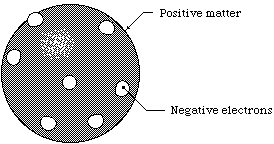
In 1897 J.J. Thomson discovered the electron and proposed the "Rasin in Pudding" model of the atom. It showed a sphere with electrons attached on the outside in random posistions.
In 1911 Ernest Rutherford sent alpha rays from Radium through gold foil and discovered most of the rays went through the foil. He then brought him to believe that in an atom there are empty spaces between the electons and the nucleus.
In 1912 Niels Bohr stated a theory of why electrons did not spiral into the positive part of an atom and stated that electrons occupy shells around the nucleus.
Around 460 B.C.E. a Greek philosopher Democritus first questioned the existence of atoms. He thought the atom was a sphere with barbs on the surface that held it together with other atoms. Not until about the 1800's though did the idea of the atom become revised. John Dalton was the first modern scientist to look at the fact that matter was made up of atoms.
Subscribe to:
Post Comments (Atom)


No comments:
Post a Comment